Carrier Screening Market Outlook: Size, Share, Trends & Growth Analysis (2024-2029)
The market report presents a thorough analysis segmented by Type (Targeted Disease Carrier Screening, Expanded Carrier Screening, Customized Panel Testing, Predesigned Panel Testing); by End User (Hospitals and Medical Centers, Clinical Laboratories, Fertility Clinics, Others); by Geography (North America, South America, Asia Pacific, Europe, The Middle East, Africa).
Outlook
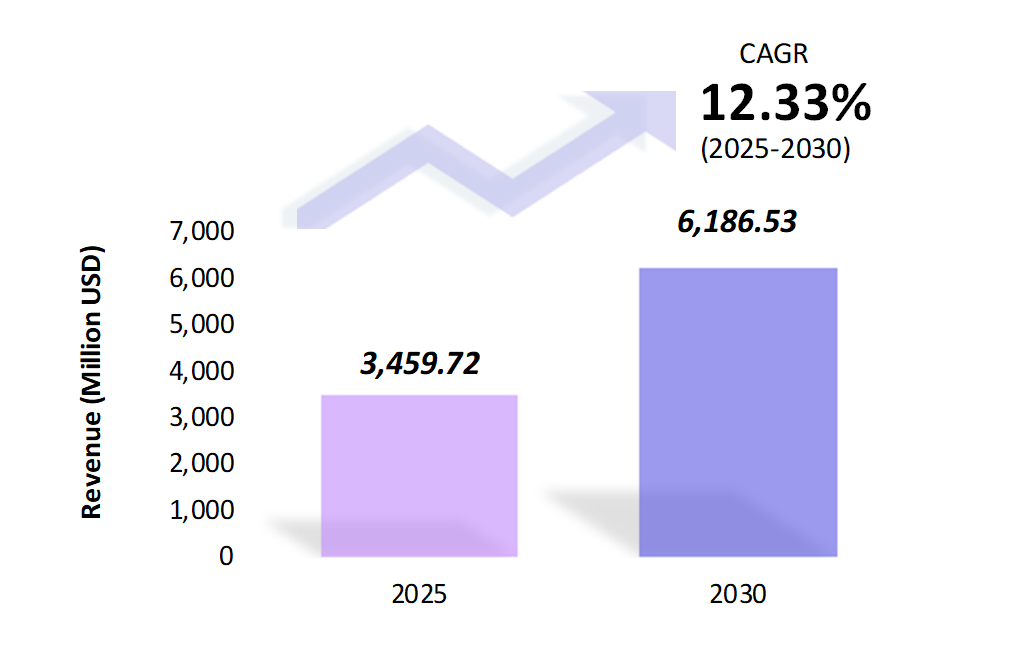
- The carrier screening market is estimated to be at USD 3,459.72 Mn in 2025 and is anticipated to reach USD 6,186.53 Mn in 2030.
- The carrier screening market is registering a CAGR of 12.33% during the forecast period 2025-2030.
- The global carrier screening market is witnessing steady growth, driven by advancements in genetic testing technologies and increasing awareness of genetic disorders. Carrier screening helps identify individuals who carry a copy of a gene mutation that can lead to inherited disorders.
Request a free sample.
Ecosystem
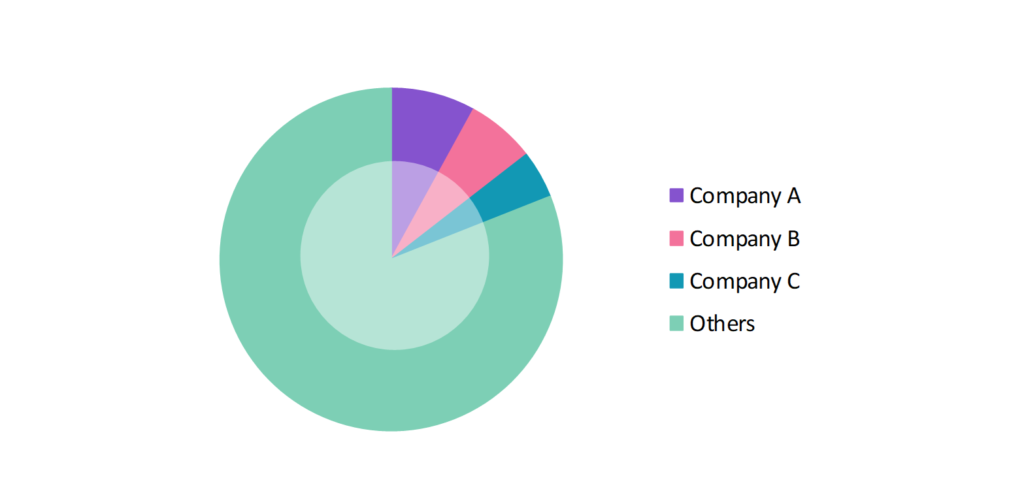
- The global carrier screening industry participants are always developing strategies to preserve a competitive advantage.
- These companies primarily use advanced sequencing platforms, non-invasive prenatal testing (NIPT) and carrier screening solutions, acquisitions, R&D, partnerships, and technological launches.
- Several important entities in the carrier screening market include Invitae Corp.; Fulgent Genetics Inc.; Thermo Fisher Scientific Inc.; Quest Diagnostics Inc.; Myriad Genetics, Inc.; and others.
Ask for customization.
Findings
| Attributes | Values |
|---|---|
| Historical Period | 2019-2023 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Market Size (2025) | USD 3,459.72 Mn |
| Market Size (2030) | USD 6,186.53 Mn |
| Growth Rate | 12.33% CAGR from 2025 to 2030 |
| Key Segments | Type (Targeted Disease Carrier Screening, Expanded Carrier Screening, Customized Panel Testing, Predesigned Panel Testing); End User (Hospitals and Medical Centers, Clinical Laboratories, Fertility Clinics, Others); Geography (North America, South America, Asia Pacific, Europe, The Middle East, Africa) |
| Key Vendors | Invitae Corp.; Fulgent Genetics Inc.; Thermo Fisher Scientific Inc.; Quest Diagnostics Inc.; Myriad Genetics, Inc. |
| Key Countries | The US; Canada; Mexico; Brazil; Argentina; Colombia; China; India; Japan; The UK; Germany; France; UAE; Saudi Arabia; Egypt; South Africa |
| Largest Market | North America |
Get a free quote.
Trends
- Technological Advancements in Genetic Testing: The development of next-generation sequencing (NGS) and microarray technology has revolutionized carrier screening, allowing for more accurate, efficient, and comprehensive testing. In 2022, Illumina introduced an updated version of its NGS technology that improves the detection of rare genetic variants, enabling more precise carrier screening.
- Integration of Artificial Intelligence in Genetic Data Analysis: The integration of AI and machine learning in carrier screening significantly enhances the speed and accuracy of data analysis. These advanced technologies can process large volumes of genetic information efficiently, allowing for quicker identification of carriers and reducing the potential for human error. As a result, healthcare providers can offer more timely and precise genetic counseling, ultimately improving patient outcomes and streamlining the decision-making process for family planning.
- Emergence of Expanded Carrier Screening Panels: There is an emphasis on expanded carrier screening panels that test for a broader range of genetic conditions. Traditional tests focused on a few conditions, but expanded panels now include hundreds of recessive disorders. For instance, in 2023, Counsyl launched an expanded carrier screening panel covering over 175 genetic conditions, providing more comprehensive screening options for prospective parents.
Speak to analyst.
Catalysts
- Rising Awareness of Genetic Disorders: Growing public awareness of the importance of genetic screening to prevent hereditary diseases drives the demand for carrier screening services. Educational campaigns by health organizations and governments are encouraging individuals to undergo carrier testing.
- Growing Use of Carrier Screening in Assisted Reproductive Technologies (ART): The integration of carrier screening into ART procedures such as in vitro fertilization (IVF) is becoming standard practice, ensuring that genetic risks are minimized for prospective parents. In 2023, IVF clinics across Asia saw a surge in demand for carrier screening as part of the pre-implantation genetic diagnosis (PGD) process, reducing the risk of passing on genetic disorders and increasing market revenue.
- Government Initiatives and Regulatory Support: Government initiatives to improve access to genetic testing are critical to driving market growth. In 2022, the U.S. government allocated funds to expand access to genetic screening services in underserved communities, focusing on early detection of hereditary diseases. Similarly, European governments are providing incentives for expanded genetic testing, accelerating the adoption of carrier screening.
Inquire before buying.
Restraints
- Ethical and Privacy Concerns: Genetic testing raises ethical concerns related to privacy, data security, and the potential for genetic discrimination. Many individuals hesitate to undergo carrier screening because they fear how their genetic data might be used or shared. This apprehension hinders participation in valuable screening programs, impacts overall public health initiatives aimed at preventing genetic disorders, and impacts revenue growth.
- High Cost of Carrier Screening: Although prices have decreased with technological advancements, carrier screening can still be expensive, particularly for expanded panels. Many insurance providers do not fully cover the costs of genetic testing, making it inaccessible for some populations.
- Regulatory Hurdles and Variability: Regulatory frameworks for genetic testing and carrier screening differ significantly between regions, posing a challenge for market players. Stringent regulations in countries like Germany and Japan, where genetic testing is heavily regulated, complicate market entry for international companies. In 2023, a regulatory review in the European Union introduced more stringent guidelines for carrier screening, delaying the approval of new screening technologies.
Personalize this research.
Hotspot
Explore purchase options.
Table of Contents
| 1. Introduction 1.1. Research Methodology 1.2. Scope of the Study 2. Market Overview / Executive Summary 2.1. Global Carrier Screening Market (2019 – 2023) 2.2. Global Carrier Screening Market (2024 – 2030) 3. Market Segmentation 3.1. Global Carrier Screening Market by Type 3.1.1. Targeted Disease Carrier Screening 3.1.2. Expanded Carrier Screening 3.1.3. Customized Panel Testing 3.1.4. Predesigned Panel Testing 3.2. Global Carrier Screening Market by End User 3.2.1. Hospitals and Medical Centers 3.2.2. Clinical Laboratories 3.2.3. Fertility Clinics 3.2.4. Others 4. Regional Segmentation 4.1. North America 4.1.1. The US 4.1.2. Canada 4.1.3. Mexico 4.2. South America 4.2.1. Brazil 4.2.2. Argentina 4.2.3. Colombia 4.2.4. Rest of South America 4.3. Asia Pacific 4.3.1. China 4.3.2. India 4.3.3. Japan 4.3.4. Rest of Asia Pacific 4.4. Europe 4.4.1. The UK 4.4.2. Germany 4.4.3. France 4.4.4. Rest of Europe 4.5. The Middle East 4.5.1. UAE 4.5.2. Saudi Arabia 4.5.3. Rest of the Middle East 4.6. Africa 4.6.1. Egypt 4.6.2. South Africa 4.6.3. Rest of Africa 5. Value Chain Analysis of the Global Carrier Screening Market 6. Porter Five Forces Analysis 6.1. Threats of New Entrants 6.2. Threats of Substitutes 6.3. Bargaining Power of Buyers 6.4. Bargaining Power of Suppliers 6.5. Competition in the Industry 7. Trends, Drivers and Challenges Analysis 7.1. Market Trends 7.1.1. Market Trend 1 7.1.2. Market Trend 2 7.1.3. Market Trend 3 7.2. Market Drivers 7.2.1. Market Driver 1 7.2.2. Market Driver 2 7.2.3. Market Driver 3 7.3. Market Challenges 7.3.1. Market Challenge 1 7.3.2. Market Challenge 2 7.3.3. Market Challenge 3 8. Opportunities Analysis 8.1. Market Opportunity 1 8.2. Market Opportunity 2 8.3. Market Opportunity 3 9. Competitive Landscape 9.1. Invitae Corp. 9.2. Fulgent Genetics, Inc. 9.3. Thermo Fisher Scientific Inc. 9.4. Quest Diagnostics Inc. 9.5. Myriad Genetics, Inc. 9.6. Company 6 9.7. Company 7 9.8. Company 8 9.9. Company 9 9.10. Company 10 |
Know the research methodology.
Carrier Screening Market – FAQs
1. What is the current size of the carrier screening market?
Ans. In 2025, the carrier screening market size is USD 3,459.72 Mn.
2. Who are the major vendors in the carrier screening market?
Ans. The major vendors in the carrier screening market are Invitae Corp.; Fulgent Genetics, Inc.; Thermo Fisher Scientific Inc.; Quest Diagnostics Inc.; Myriad Genetics, Inc.
3. Which segments are covered under the carrier screening market segments analysis?
Ans. The carrier screening market report offers in-depth insights into Type, End User, and Geography.
