Clinical Trial Supplies Market: Size, Share, Trends & Forecast (2024-2029)
The report covers a comprehensive analysis segmented by Services (Manufacturing, Packaging, Logistics), By Type (Small- Molecules, Biologic Drugs), By Therapeutics (Oncology, CVD, Infectious, Immunology), By End User (Pharmaceuticals Companies, Contract Research Organizations (CRO’s), Bio-Tech), By Geography (North America, South America, Asia Pacific, Europe, The Middle East, Africa).
Clinical Trial Supplies Market Snapshot
Clinical Trial Supplies Market Overview
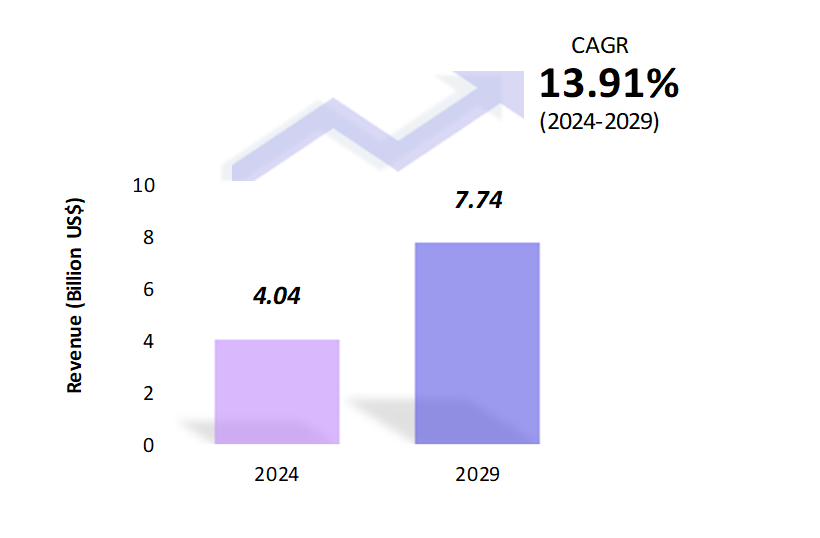
The market for clinical trials is projected to grow from $4.04 billion (about $12 per person in the US) in 2024 to $7.74 billion (about $24 per person in the US) in 2029. Between 2024 and 2029, the global clinical trial market is expected to grow at a compound annual growth rate (CAGR) of 13.91%.
As a vital link between patient care and scientific research, the clinical trial market is essential to the creation of novel medical treatments and cures. It includes a wide range of stakeholders, including academic institutions, regulatory agencies, patients, pharmaceutical and biotechnology corporations, and contract research organizations (CROs). The market for clinical trials has grown steadily in recent years due to several causes, including the rise in the prevalence of uncommon and chronic diseases, improvements in medical technology, and changing regulatory environments and it is characterized by a move toward targeted therapy catered to the unique characteristics of each patient and a growing emphasis on customized medicine.
Clinical trial practices are changing as a result of technological innovation, including the use of decentralized trial models, artificial intelligence, and digital health systems. Data analytics, patient engagement, and trial efficiency are improving due to these developments. Notwithstanding these advantages, the clinical trial industry still must contend with issues like patient acquisition and retention, complicated regulations, growing expenses, and worries about the quality of data. To overcome these obstacles, stakeholders must work together, adopt creative solutions, and continue to adjust to shifting market conditions. Since clinical trials are essential to the advancement of medical science and the improvement of patient outcomes, the market is expected to develop and innovate going forward.
Clinical Trial Supplies Market Coverage
| Historical & Forecast Period | 2018-2029 |
| Base Year | 2023 |
| Forecast Period | 2024-2029 |
| Units | Billion US$ |
| Segments | By Services, By Type, By Therapeutics, End User |
| Geographies | North America, South America, Asia Pacific, Europe, The Middle East, Africa |
| Key Vendors |
IQVIA Inc., Thermo Fisher Scientific Inc., Catalent, Inc., Eurofins Scientific SE, Parexel International (MA) Corp. |
Key Geographies of Clinical Trial Supplies Market, 2023
Porter’s 5 Forces Analysis of Clinical Trial Supplies Market

Clinical Trial Supplies Market Trends
The clinical trial market has experienced significant trends in recent years, reflecting advancements in technology, changes in regulatory landscapes, and shifts in healthcare priorities. One prominent trend is the increasing globalization of clinical trials. Companies are conducting trials in diverse geographic regions to access larger patient pools, reduce costs, and expedite the drug development process. Additionally, there is a discernible increase in the use of decentralized and virtual clinical trials. The COVID-19 pandemic, which brought attention to the need for remote monitoring and patient-centric techniques, has advanced this tendency. Virtual trials are more convenient for participants, which may lower logistical costs and increase recruitment and retention rates. Furthermore, real-world evidence (RWE) and patient-centricity are becoming more important in clinical trial design.
Stakeholders understand that including patient perspectives and outcomes representative of everyday life is crucial to producing more significant findings and wiser healthcare decisions. Additionally, several clinical trial processes are being revolutionized by the integration of artificial intelligence (AI) and machine learning (ML) technology, from patient recruitment and data analysis to predictive modeling and customized medicine. These technologies allow customized therapies based on unique patient features, improving efficiency, accuracy, and scalability. Regulatory bodies are also taking steps to improve openness and expedite trial procedures.
Two examples of these efforts are the EMA’s Adaptive Pathways project and the FDA’s use of real-time data monitoring. These initiatives seek to shorten the time frames for medication development without sacrificing standards for efficacy or safety. Improvements in efficiency, efficacy, and patient outcomes are being driven by the clinical trial market’s evolution toward increased global collaboration, patient-centricity, technological integration, and regulatory innovation.
Clinical Trial Supplies Market Driving Factors
Numerous key factors impact the clinical trial market, determining its growth and direction. The rising incidence of uncommon and chronic illnesses is one major factor driving the need for novel medications and treatments. Pharmaceutical and biotechnology businesses are forced to invest in clinical research to extend their product portfolios and meet unmet medical needs as the worldwide burden of diseases rises. Furthermore, new developments in clinical trial methodology are being fueled by technological breakthroughs, especially in fields like genomics, biomarkers, and digital health. These technological advancements improve trial efficiency and results by enabling more accurate patient categorization, customized treatment plans, and remote monitoring.
A noteworthy additional motivator is the dynamic regulatory environment. Global regulatory bodies are putting policies into place to expedite trial procedures, shorten approval periods, and promote innovation. For instance, corporations are encouraged to invest in clinical research for specialized indications by accelerated paths for orphan pharmaceuticals and breakthrough therapies. Globalization has also been a major factor, with businesses conducting trials across many geographies to reach a wider patient base, make use of local knowledge, and more effectively manage regulatory variations. The market for clinical trials is shaped and propelled forward in medical research and medication development by several variables, including illness prevalence, technological innovation, regulatory measures, the need for real-world evidence, and globalization.
Clinical Trial Supplies Market Challenges
The efficiency and efficacy of the clinical trial supplies market are impacted by several issues. The intricacy of worldwide regulatory regulations is one major obstacle. Regulations on clinical trial materials differ between nations, which might result in more administrative work and delays in approvals. Furthermore, as research gets more focused and individualized, there is an increasing need for specialty and personalized trial supplies. This makes manufacturing and forecasting difficult, particularly for small-scale studies or specialized indications. Obstacles related to logistics can cause difficulties, especially for overseas trials. It can be challenging to guarantee the timely delivery of commodities while preserving their integrity and quality during transit, particularly in areas with inadequate infrastructure or unstable political environments.
In addition, the swift progress of technology and inventiveness in clinical research poses difficulties in modifying supply chain procedures and frameworks to suit novel demands and guidelines. In conclusion, sponsors and suppliers within the clinical trial supplies market continually grapple with cost pressures and budget constraints, necessitating the implementation of cost-containment strategies that uphold quality and compliance standards. Considering these challenges, addressing them effectively requires collaboration among stakeholders, innovative problem-solving approaches, and a proactive stance toward navigating evolving legal and technological landscapes.
Clinical Trial Supplies Market – Key Industry News
- In March 2024, Veeda Clinical Research Ltd., a full-service contract research organization (CRO), acquired Health Data Specialists ‘’Heads’’, a privately held European CRO specializing in conducting clinical trials in oncology. Heads has an operational presence in 25 strategic locations across Europe, North America, and the Asia Pacific region. The acquisition expands Veeda’s global reach and adds integrated capabilities for contract research services from discovery to clinical development extending to post-commercial launch.
- In February 2024, FedEx Corp., has unveiled its FedEx Life Science Centre in Mumbai, setting a benchmark in the clinical trial supply chain in India and globally.
- In July 2023, Signant Health, the leader in evidence generation for modern clinical trials, announced expanded flexibility and capabilities for its leading Signant SmartSignals Supplies software, empowering small and mid-size customers to experience the advantages of technology-driven clinical supply management.
Clinical Trial Supplies Market Competitive Landscape
The Global Clinical Trial Supplies market is highly competitive, and it is marked by the presence of many prominent players competing for a larger market share. The participants in the global clinical trial supplies industry are always developing their strategies to preserve a competitive advantage. . Companies primarily use acquisitions, R&D, partnerships, and technological launches. Several important entities in the clinical trial supplies market include IQVIA Inc., Thermo Fisher Scientific Inc., Catalent, Inc., Eurofins Scientific SE, Parexel International (MA) Corp., and others.
In addition, innovation is driven by changing consumer needs, security concerns, and emerging technologies. The market dynamics are shaped by key factors such as the adoption of contactless payment methods, biometric authentication, and seamless interaction with digital banking platforms.
Clinical Trial Supplies Market Company Share Analysis, 2023 (%)

Clinical Trial Supplies Market – Key Companies

Reason to Buy from us
Table of Contents
| 1. Introduction |
|---|
| 1.1. Research Methodology |
| 1.2. Scope of the Study |
| 2. Market Overview / Executive Summary |
| 2.1. Global Clinical Trial Supplies Market (2018 – 2022) |
| 2.2. Global Clinical Trial Supplies Market (2023 – 2029) |
| 3. Market Segmentation |
| 3.1. Global Ceramics Market by Services |
| 3.1.1. Manufacturing |
| 3.1.2. Packaging |
| 3.1.3. Logistics |
| 3.2. Global Clinical Trial Supplies Market by Type |
| 3.2.1. Small-Molecules |
| 3.2.2. Biologic Drugs |
| 3.3. Global Clinical Trial Supplies Market by Therapeutics |
| 3.3.1. Oncology |
| 3.3.2. CVD |
| 3.3.3. Infectious |
| 3.3.4. Immunology |
| 3.4. Global Clinical Trial Supplies by End User |
| 3.4.1. Pharmaceuticals Companies |
| 3.4.2. Contract Research Organizations (CRO’s) |
| 3.4.3. Bio Tech |
| 4. Regional Segmentation |
| 4.1. North America |
| 4.1.1. The U.S |
| 4.1.2. Canada |
| 4.1.3. Mexico |
| 4.2. South America |
| 4.2.1. Brazil |
| 4.2.2. Argentina |
| 4.2.3. Colombia |
| 4.2.4. Chile |
| 4.2.5. Rest of South America |
| 4.3. Asia Pacific |
| 4.3.1. China |
| 4.3.2. India |
| 4.3.3. Japan |
| 4.3.4. South Korea |
| 4.3.5. Rest of Asia Pacific |
| 4.4. Europe |
| 4.4.1. UK |
| 4.4.2. Germany |
| 4.4.3. Italy |
| 4.4.4. France |
| 4.4.5. Spain |
| 4.4.6. Rest of Europe |
| 4.5. The Middle East |
| 4.5.1. Turkey |
| 4.5.2. UAE |
| 4.5.3. Saudi Arabia |
| 4.5.4. Rest of the Middle East |
| 4.6. Africa |
| 4.6.1. Egypt |
| 4.6.2. South Africa |
| 4.6.3. Rest of Africa |
| 5. Value Chain Analysis of the Global Clinical Trial Supplies Market |
| 6. Porter Five Forces Analysis |
| 6.1. Threats of New Entrants |
| 6.2. Threats of Substitutes |
| 6.3. Bargaining Power of Buyers |
| 6.4. Bargaining Power of Suppliers |
| 6.5. Competition in the Industry |
| 7. Trends, Drivers and Challenges Analysis |
| 7.1. Market Trends |
| 7.1.1. Market Trend 1 |
| 7.1.2. Market Trend 2 |
| 7.1.3. Market Trend 3 |
| 7.1.4. Market Trend 4 |
| 7.1.5. Market Trend 5 |
| 7.2. Market Drivers |
| 7.2.1. Market Driver 1 |
| 7.2.2. Market Driver 2 |
| 7.2.3. Market Driver 3 |
| 7.2.4. Market Driver 4 |
| 7.2.5. Market Driver 5 |
| 7.3. Market Challenges |
| 7.3.1. Market Challenge 1 |
| 7.3.2. Market Challenge 2 |
| 7.3.3. Market Challenge 3 |
| 7.3.4. Market Challenge 4 |
| 7.3.5. Market Challenge 5 |
| 8. Regulatory Landscape |
| 9. Competitive Landscape |
| 9.1. IQVIA Inc. |
| 9.2. Thermo Fisher Scientific Inc. |
| 9.3. Catalent, Inc. |
| 9.4. Eurofins Scientific SE |
| 9.5. Parexel International (MA) Corp. |
| 9.6. Company 6 |
| 9.7. Company 7 |
| 9.8. Company 8 |
| 9.9. Company 9 |
| 9.10. Company 10 |
Clinical Trial Supplies Market – Frequently Asked Questions (FAQs)
What is the current size of the global clinical trial supplies market?
The global Clinical Trial market is estimated to be at $4.04 Bn in 2024 and is anticipated to reach $7.74 Bn in 2029.
Who are the major vendors in the global clinical trial supplies market?
The major vendors in the global clinical trial supplies market are IQVIA Inc., Thermo Fisher Scientific Inc., Catalent, Inc., Eurofins Scientific SE, Parexel International (MA) Corp.
Which segments are covered under the global clinical trial supplies market segments analysis?
This report offers in-depth insights into each by services, by type, by therapeutics area, end user.
