In-vitro Transcription Templates Market Insights: Size, Share, Growth Analysis & Forecast (2024-2029)
The market report provided a comprehensive analysis segmented by Disease Type (Cancer, Infectious Disease, Genetic Disease, Others); by Treatment Type (Vaccine, Therapeutic); by End User (Pharmaceutical Companies, Academic Institutes, Research Institutes, Others); by Geography (North America, South America, Asia Pacific, Europe, The Middle East, Africa).
Outlook
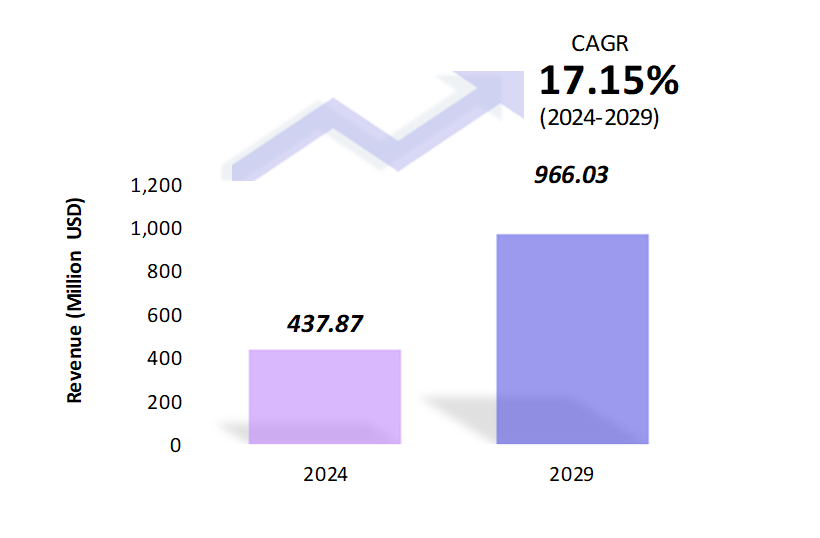
- The in-vitro transcription templates market is estimated to be at USD 437.87 Mn in 2024 and is anticipated to reach USD 966.03 Mn in 2029.
- The in-vitro transcription templates market is registering a CAGR of 17.15% during the forecast period of 2024-2029.
- In-vitro transcription (IVT) templates are DNA sequences used to synthesize RNA in a controlled laboratory environment. The market for IVT templates is expanding due to the growing demand for mRNA vaccines, gene therapy, and RNA-based research. The flexibility and efficiency of IVT templates make them indispensable in modern biotechnology.
Request a free sample.
Ecosystem
- The participants in the global in-vitro transcription templates industry are always developing their strategies to preserve a competitive advantage.
- Companies are focusing on enhancing the efficiency and reliability of their IVT templates. For instance, TriLink Biotechnologies has developed CleanCap technology, which improves the capping efficiency of mRNA transcripts, making them more suitable for therapeutic applications.
- Several important entities in the in-vitro transcription templates market include LGC Genomics Ltd.; Danaher Corp.; Takara Bio Inc.; Agilent Technologies, Inc.; New England Biolabs, Inc.; and others.
Ask for customization.
Findings
| Attributes | Values |
|---|---|
| Historical Period | 2018-2022 |
| Base Year | 2023 |
| Forecast Period | 2024-2029 |
| Market Size (2024) | USD 437.87 Mn |
| Market Size (2029) | USD 966.03 Mn |
| Growth Rate | 17.15% CAGR from 2024 to 2029 |
| Key Segments | Disease Type (Cancer, Infectious Diseases, Genetic Disease, Others); Treatment Type (Vaccine, Therapeutic); End User (Pharmaceutical Companies, Academic Institutes, Research Institutes, Others); Geography (North America, South America, Asia Pacific, Europe, The Middle East, Africa) |
| Key Vendors | LGC Genomics Ltd.; Danaher Corp.; Takara Bio Inc.; Agilent Technologies, Inc.; New England Biolabs, Inc. |
| Key Countries | The US; Canada; Mexico; Brazil; Argentina; Colombia; Chile; China; India; Japan; South Korea; The UK; Germany; Italy; France; Spain; Turkey; UAE; Saudi Arabia; Egypt; South Africa |
| Largest Market | North America |
Get a free quote.
Trends
- Leveraging scRNA-seq: The use of single-cell RNA sequencing (scRNA-seq) is emerging as a significant trend in the in-vitro transcription templates market. By providing high-resolution expression profiles of individual cells, scRNA-seq enables the creation of transcription templates that are precisely tailored to specific cell types or states.
- Customization of IVT Templates for Specialized Applications: Customization of IVT templates to meet specific research and therapeutic needs is becoming increasingly important. Companies are now offering tailored IVT templates designed to cater to the unique RNA sequences required for niche applications. Aldevron, a company specializing in custom IVT template production, collaborates with biotech firms to develop personalized RNA sequences for specific therapeutic targets.
- Integration of IVT Templates with High-Throughput Screening: The integration of IVT templates with high-throughput screening technologies is becoming more prevalent. This allows researchers to rapidly test large numbers of RNA sequences for potential therapeutic effects. Horizon Discovery has integrated IVT templates into its high-throughput Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) screening platform, enabling faster identification of RNA sequences that could serve as therapeutic candidates in gene editing applications.
Speak to analyst.
Catalysts
- Surge in mRNA Vaccine Development: The success of mRNA vaccines, especially during the COVID-19 pandemic, has driven significant interest and investment in IVT templates. Pfizer Inc. as well as Moderna have extensively utilized IVT templates in the development and production of their mRNA vaccines, which leads to increased demand for these templates in vaccine research and manufacturing.
- Growth in Synthetic Biology: Synthetic biology, which involves designing and constructing new biological parts, devices, and systems, relies on IVT templates for creating RNA components. The expansion of this field is driving the demand for IVT templates. For instance, Ginkgo Bioworks, a synthetic biology company, uses IVT templates in its engineered organisms, which are designed for various industrial applications, including pharmaceuticals and biofuels.
- Rising Demand for Personalized Medicine: Personalized medicine, which tailors treatments to individual genetic profiles, often involves the use of IVT templates in creating patient-specific RNA sequences. This trend is expected to further fuel market growth. BioNTech’s personalized cancer vaccines use IVT templates to produce RNA sequences tailored to each patient’s tumor profile, enabling a more targeted and effective treatment approach.
Inquire before buying.
Restraints
- Complexity of IVT Template Design: Designing IVT templates that accurately and efficiently produce the desired RNA sequences can be technically challenging, requiring specialized expertise and tools. Smaller biotech firms may struggle with the technical demands of IVT template design, limiting their ability to compete with larger, well-funded companies in RNA-based therapeutics.
- High Production Costs: The production of high-quality IVT templates involves significant costs, which can be a barrier for smaller companies or research institutions. Startups in the RNA therapeutics space often face financial challenges in scaling up the production of IVT templates due to the high costs associated with maintaining quality and efficiency.
- Regulatory Hurdles: The use of IVT templates in therapeutic applications, particularly in gene therapy and vaccines, must comply with stringent regulatory standards, which can slow down product development and market entry. CureVac Company has faced delays in bringing its RNA-based therapeutics to market due to the rigorous regulatory approval process required for products involving IVT templates.
Personalize this research.
Hotspot
Explore purchase options.
Table of Contents
| 1. Introduction 1.1. Research Methodology 1.2. Scope of the Study 2. Market Overview / Executive Summary 2.1. Global In-vitro Transcription Templates Market (2018 – 2022) 2.2. Global In-vitro Transcription Templates Market (2023 – 2029) 3. Market Segmentation 3.1. Global In-vitro Transcription Templates Market by Disease Type 3.1.1. Cancer 3.1.2. Infectious Disease 3.1.3. Genetic Disease 3.1.4. Others 3.2. Global In-vitro Transcription Templates Market by Treatment Type 3.2.1. Vaccine 3.2.2. Therapeutic 3.3. Global In-vitro Transcription Templates Market by End User 3.3.1. Pharmaceutical Companies 3.3.2. Academic Institutes 3.3.3. Research Institutes 3.3.4. Others 4. Regional Segmentation 4.1. North America 4.1.1. The US 4.1.2. Canada 4.1.3. Mexico 4.2. South America 4.2.1. Brazil 4.2.2. Argentina 4.2.3. Colombia 4.2.4. Chile 4.2.5. Rest of South America 4.3. Asia Pacific 4.3.1. China 4.3.2. India 4.3.3. Japan 4.3.4. South Korea 4.3.5. Rest of Asia Pacific 4.4. Europe 4.4.1. The UK 4.4.2. Germany 4.4.3. Italy 4.4.4. France 4.4.5. Spain 4.4.6. Rest of Europe 4.5. The Middle East 4.5.1. Turkey 4.5.2. UAE 4.5.3. Saudi Arabia 4.5.4. Rest of the Middle East 4.6. Africa 4.6.1. Egypt 4.6.2. South Africa 4.6.3. Rest of Africa 5. Value Chain Analysis of the Global In-vitro Transcription Templates Market 6. Porter Five Forces Analysis 6.1. Threats of New Entrants 6.2. Threats of Substitutes 6.3. Bargaining Power of Buyers 6.4. Bargaining Power of Suppliers 6.5. Competition in the Industry 7. Trends, Drivers and Challenges Analysis 7.1. Market Trends 7.1.1. Market Trend 1 7.1.2. Market Trend 2 7.1.3. Market Trend 3 7.2. Market Drivers 7.2.1. Market Driver 1 7.2.2. Market Driver 2 7.2.3. Market Driver 3 7.3. Market Challenges 7.3.1. Market Challenge 1 7.3.2. Market Challenge 2 7.3.3. Market Challenge 3 8. Opportunities Analysis 8.1. Market Opportunity 1 8.2. Market Opportunity 2 8.3. Market Opportunity 3 9. Competitive Landscape 9.1. LGC Genomics Ltd. 9.2. Danaher Corp. 9.3. Takara Bio Inc. 9.4. Agilent Technologies, Inc. 9.5. New England Biolabs, Inc. 9.6. Company 6 9.7. Company 7 9.8. Company 8 9.9. Company 9 9.10. Company 10 |
Know the research methodology.
In-vitro Transcription Templates Market – FAQs
1. What is the current size of the in-vitro transcription templates market?
Ans. In 2024, the in-vitro transcription templates market size is $437.87 Mn.
2. Who are the major vendors in the in-vitro transcription templates market?
Ans. The major vendors in the in-vitro transcription templates market are LGC Genomics Ltd.; Danaher Corp.; Takara Biotechnology Co.; Agilent Technologies, Inc.; New England Biolabs, Inc.
3. Which segments are covered under the in-vitro transcription templates market segments analysis?
Ans. The in-vitro transcription templates market report offers in-depth insights into Disease Type, Treatment Type, End User, and Geography.
