Pediatric Drugs Market: Size, Share, Trends & Forecast (2024-2029)
The market report offers a detailed analysis segmented by Type (Respiratory Disorder Drugs, Autoimmune Disorder Drugs, Gastrointestinal Drugs, Cardiovascular Drugs, Others); by Route of Administration (Oral, Topical, Parenteral, Others); by Geography (North America, South America, Asia Pacific, Europe, The Middle East, Africa).
Outlook
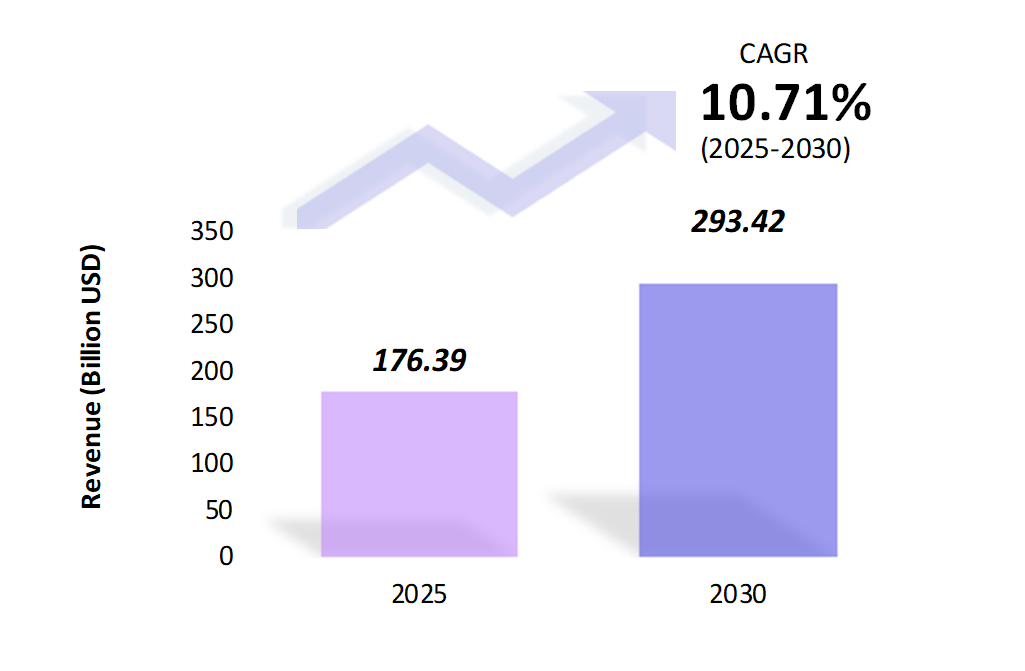
- The pediatric drugs market is estimated to be at USD 176.39 Bn in 2025 and is anticipated to reach USD 293.42 Bn in 2030.
- The pediatric drugs market is registering a CAGR of 10.71% during the forecast period 2025-2030.
- The global pediatric drugs market is witnessing steady growth, fueled by rising pediatric populations, increased awareness about child health, and advancements in medical treatments specifically tailored for children.
Request a free sample.
Ecosystem
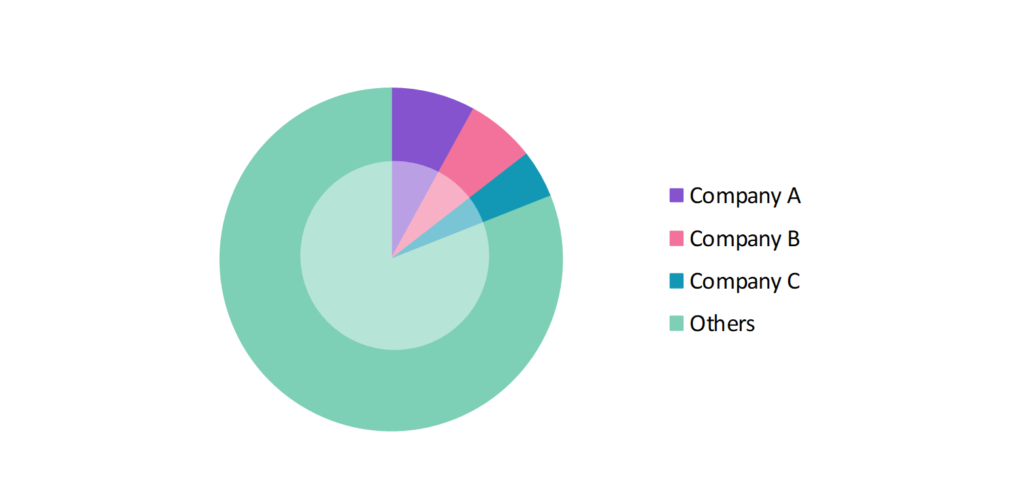
- The participants in the global pediatric drugs industry are always developing their strategies to preserve a competitive advantage.
- These companies primarily use acquisitions, investments, research & developments, partnerships, and technological launches.
- Several important entities in the pediatric drugs market include BioMarin Pharmaceutical Inc.; Amgen Inc.; PTC Therapeutics, Inc.; Jazz Pharmaceuticals Plc; Gilead Sciences, Inc.; and others.
Ask for customization.
Findings
| Attributes | Values |
|---|---|
| Historical Period | 2019-2023 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Market Size (2025) | USD 176.39 Bn |
| Market Size (2030) | USD 293.42 Bn |
| Growth Rate | 10.71% CAGR from 2025 to 2030 |
| Key Segments | Type (Respiratory Disorder Drugs, Autoimmune Disorder Drugs, Gastrointestinal Drugs, Cardiovascular Drugs, Others); Route of Administration (Oral, Topical, Parenteral, Others); Geography (North America, South America, Asia Pacific, Europe, The Middle East, Africa) |
| Key Vendors | BioMarin Pharmaceutical Inc.; Amgen Inc.; PTC Therapeutics, Inc.; Jazz Pharmaceuticals Plc; Gilead Sciences, Inc. |
| Key Countries | The US; Canada; Mexico; Brazil; Argentina; Colombia; China; India; Japan; The UK; Germany; France; UAE; Saudi Arabia; Egypt; South Africa |
| Largest Market | North America |
Get a free quote.
Trends
- Emergence of Personalized Pediatric Medicines: Advances in genomics and biotechnology enable the development of personalized medications for children. By 2023, research centers have increasingly focused on tailoring treatments to pediatric patients’ unique genetic profiles, such as the European Medicines Agency’s initiative on pharmacogenomics for pediatric drugs.
- Use of Digital Health Tools in Pediatric Care: Digital health tools, such as wearables and remote monitoring, are increasingly integrated into pediatric care, particularly for chronic diseases like asthma and diabetes. These technologies help monitor young patients effectively and provide data for timely interventions.
- Advances in Pediatric-Specific Drug Formulations: Pharmaceutical companies are increasingly developing pediatric-friendly drug formulations, such as chewable and liquid medicines, which make administration more accessible and improve adherence among young patients. For example, researchers at Debre Berhan University in Ethiopia have been actively exploring 3DP technology to optimize pediatric dosage regimens and enhance individualized therapy for young patients.
Speak to analyst.
Catalysts
- Increasing Prevalence of Pediatric Diseases and Disorders: The increasing prevalence of pediatric chronic conditions like asthma, diabetes, and obesity is leading to a heightened demand for medications specifically designed for children. This trend underscores the need for age-appropriate formulations and dosing to ensure safety and effectiveness, as well as the importance of addressing the unique health challenges faced by younger populations.
- Increased Awareness and Demand for Child Health and Wellness: Rising awareness of preventive healthcare and the critical role of early intervention for childhood illnesses is significantly boosting the pediatric drug market. Parents and healthcare providers are increasingly prioritizing proactive measures, leading to a greater demand for treatments that can prevent complications and promote healthier outcomes in children. This shift emphasizes the importance of accessible and effective pediatric care.
- Government Initiatives and Regulatory Incentives: Governments worldwide are implementing policies to encourage pediatric drug development, including funding for research and tax incentives. The US FDA’s pediatric rare disease priority review voucher program, extended in 2022, incentivizes companies to invest in treatments for rare pediatric conditions.
Inquire before buying.
Restraints
- Challenges in Drug Dosage and Administration: Creating age-appropriate dosage forms for children is a complex task due to the significant variations in their absorption and metabolism compared to adults. These differences make achieving the right balance of efficacy and safety challenging, necessitating careful consideration of formulation and dosing to ensure that pediatric medications meet the unique physiological needs of younger patients.
- Adverse Drug Reactions in Pediatric Patients: Pediatric patients are particularly susceptible to adverse drug reactions, which complicate the drug development process and hinder market acceptance. This vulnerability necessitates rigorous testing and safety evaluations to ensure that medications are not only practical but also safe for younger populations, impacting both the timeline and costs associated with bringing pediatric drugs to market.
- Stringent Regulatory Requirements and Compliance Costs: Pediatric drug approval involves strict regulatory compliance and extended testing phases to ensure safety, increasing time and cost. The FDA’s heightened safety standards in 2023 added to the challenges for pharmaceutical companies trying to launch new pediatric drugs quickly.
Personalize this research.
Hotspot
Explore purchase options.
Table of Contents
| 1. Introduction 1.1. Research Methodology 1.2. Scope of the Study 2. Market Overview / Executive Summary 2.1. Global Pediatric Drugs Market (2019 – 2023) 2.2. Global Pediatric Drugs Market (2024 – 2030) 3. Market Segmentation 3.1. Global Pediatric Drugs Market by Type 3.1.1. Respiratory Disorder Drugs 3.1.2. Autoimmune Disorder Drugs 3.1.3. Gastrointestinal Drugs 3.1.4. Cardiovascular Drugs 3.1.5. Others 3.2. Global Pediatric Drugs Market by Route of Administration 3.2.1. Oral 3.2.2. Topical 3.2.3. Parenteral 3.2.4. Others 4. Regional Segmentation 4.1. North America 4.1.1. The US 4.1.2. Canada 4.1.3. Mexico 4.2. South America 4.2.1. Brazil 4.2.2. Argentina 4.2.3. Colombia 4.2.4. Rest of South America 4.3. Asia Pacific 4.3.1. China 4.3.2. India 4.3.3. Japan 4.3.4. Rest of Asia Pacific 4.4. Europe 4.4.1. The UK 4.4.2. Germany 4.4.3. France 4.4.4. Rest of Europe 4.5. The Middle East 4.5.1. UAE 4.5.2. Saudi Arabia 4.5.3. Rest of the Middle East 4.6. Africa 4.6.1. Egypt 4.6.2. South Africa 4.6.3. Rest of Africa 5. Value Chain Analysis of the Global Pediatric Drugs Market 6. Porter Five Forces Analysis 6.1. Threats of New Entrants 6.2. Threats of Substitutes 6.3. Bargaining Power of Buyers 6.4. Bargaining Power of Suppliers 6.5. Competition in the Industry 7. Trends, Drivers and Challenges Analysis 7.1. Market Trends 7.1.1. Market Trend 1 7.1.2. Market Trend 2 7.1.3. Market Trend 3 7.2. Market Drivers 7.2.1. Market Driver 1 7.2.2. Market Driver 2 7.2.3. Market Driver 3 7.3. Market Challenges 7.3.1. Market Challenge 1 7.3.2. Market Challenge 2 7.3.3. Market Challenge 3 8. Opportunities Analysis 8.1. Market Opportunity 1 8.2. Market Opportunity 2 8.3. Market Opportunity 3 9. Competitive Landscape 9.1. BioMarin Pharmaceutical Inc. 9.2. Amgen Inc. 9.3. PTC Therapeutics, Inc. 9.4. Jazz Pharmaceuticals Plc 9.5. Gilead Sciences, Inc. 9.6. Company 6 9.7. Company 7 9.8. Company 8 9.9. Company 9 9.10. Company 10 |
Know the research methodology.
Pediatric Drugs Market – FAQs
1. What is the current size of the pediatric drugs market?
Ans. In 2025, the pediatric drugs market size is USD 176.39 Bn.
2. Who are the major vendors in the pediatric drugs market?
Ans. The major vendors in the pediatric drugs market are BioMarin Pharmaceutical Inc.; Amgen Inc.; PTC Therapeutics, Inc.; Jazz Pharmaceuticals Plc; Gilead Sciences, Inc.
3. Which segments are covered under the pediatric drugs market segments analysis?
Ans. The pediatric drugs market report offers in-depth insights into Type, Route of Administration, and Geography.
